The Danish physicist Niels Henrik David Bohr was recognized with the Nobel Prize in Physics in 1922 for his fundamental contributions to the study of quantum theory and atomic structure. In addition to being a scientist, Bohr was a philosopher and an advocate for it. Bohr created the Bohr model of the atom, in which he argued that electron energy levels are discrete and that electrons orbit the atomic nucleus in stable orbits but have the ability to move between them. The fundamental ideas of the Bohr model have not changed even though other models have taken their place. He developed the complementarity principle, which states that objects can be independently analyzed in terms of contradictory characteristics, such as acting as a wave or a stream of particles. In both physics and philosophy, Bohr’s thought was dominated by the idea of complementarity.
Bohr’s Model
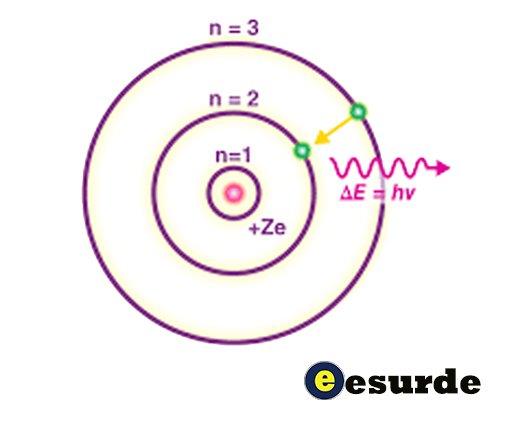
The Bohr model, also known as the Rutherford-Bohr model, was proposed by Niels Bohr and Ernest Rutherford in 1913 and depicts an atomic system with an orbiting system of electrons surrounding a small, dense nucleus. This arrangement is similar to that of the Solar System, except that electrostatic forces act as the forces of attraction rather than gravity. It followed the solar system Joseph Larmor model (1897), cubical model (1902), Saturnian model (1904) by Hantaro Nagaoka, the plum pudding model (1904), the Rutherford model (1911), the quantum Arthur Haas model (1910), and the nuclear quantum model by John William Nicholson (1912). The primary change from the Rutherford model of 1911 was the Arthur Haas and Nicholson’s new quantum physical interpretation, which abandoned any attempt to be consistent with classical physics radiation.
The postulates of Bohr’s model of an atom
- The orbit, also known as the stationary state of the shell, is the path in which electrons travel as they orbit an atom’s nucleus.
- The different energy levels in the shells are represented by the letters K, L, M, and N.
- The electron does not absorb or release energy while it is in an orbit.
- The electron can only move in an orbit whose angular momentum is quantized, meaning that the electron’s angular momentum is an integral multiple of h/2π .
